The opioid crisis stands as one of the most devastating public health catastrophes in American history, claiming over 800,000 lives since 1999 and continuing to kill nearly 80,000 people annually. At the heart of this epidemic lies a story of corporate greed, regulatory failure, and medical malpractice that transformed prescription painkillers from legitimate medical tools into instruments of mass addiction. The overprescription of OxyContin and other opioid-based painkillers didn’t just contribute to this crisis—it fundamentally created it through decades of deceptive marketing, aggressive promotion, and systematic manipulation of medical practice.
The Origins of a Manufactured Crisis
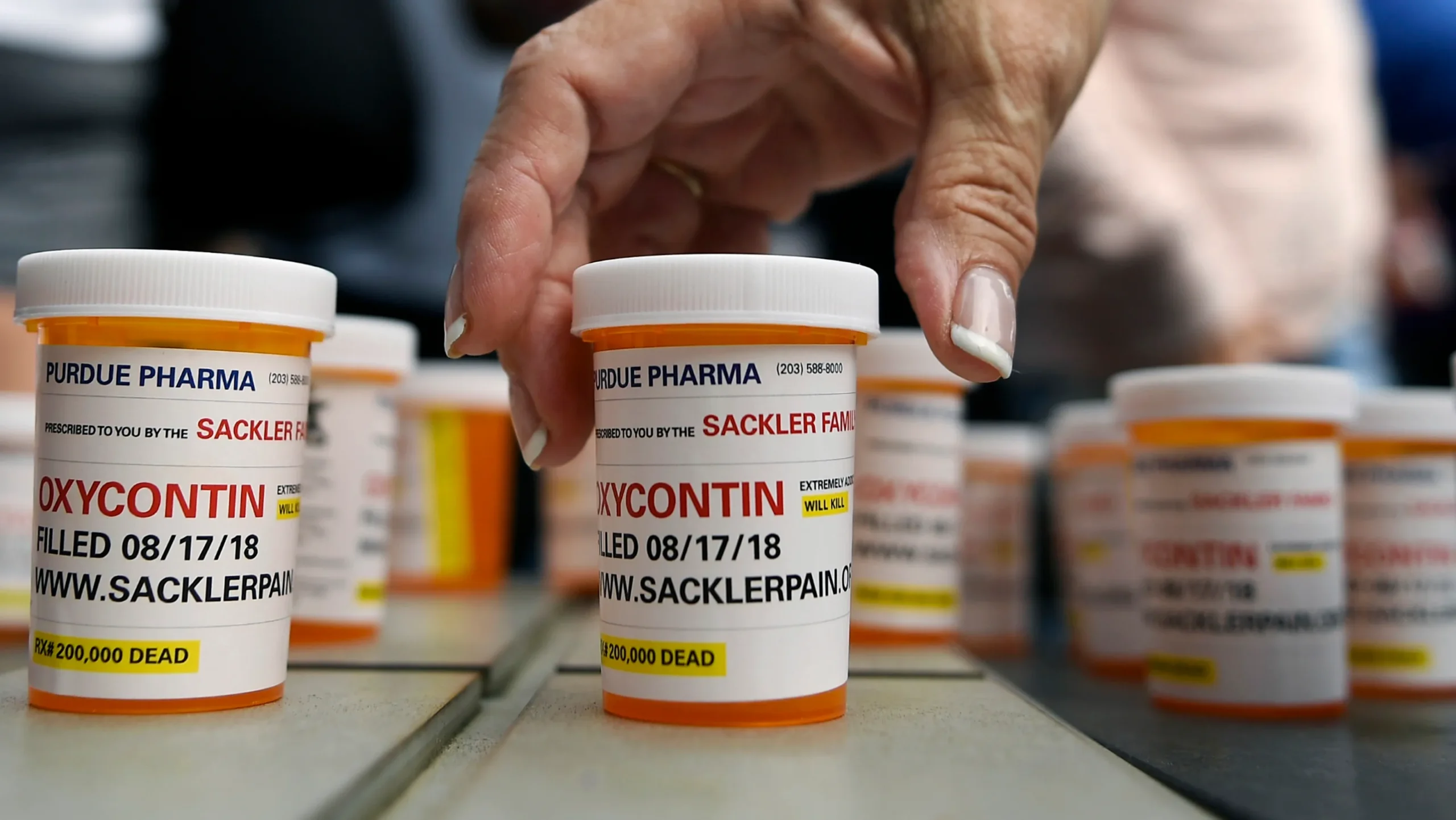
The opioid crisis emerged not from a natural disease outbreak but from what a federal judge accurately described as “a man-made plague, 20 years in the making”. When Purdue Pharma introduced OxyContin in 1996, it launched an unprecedented marketing campaign that fundamentally altered how America approached pain treatment. Sales grew explosively from $48 million in 1996 to almost $1.1 billion in 2000, making OxyContin a blockbuster drug through aggressive promotion rather than superior efficacy.
The remarkable commercial success of OxyContin was achieved despite research showing it was comparable in efficacy and safety to other available opioids. What set OxyContin apart was not its medical superiority but the scope and intensity of its marketing campaign, which spent approximately 6 to 12 times more on promotion than competitors during its first six years. This unprecedented promotional spending created artificial demand for a controlled substance with known addiction potential, prioritizing profits over patient safety (NIH).
Deceptive Marketing and False Claims
Central to Purdue Pharma’s strategy was a systematic effort to minimize the risk of addiction in opioid use for chronic non-cancer pain. The company trained its sales representatives to carry the message that the risk of addiction was “less than one percent,” citing studies that examined only acute pain situations rather than the chronic pain applications they were promoting. This fundamental misrepresentation formed the cornerstone of a marketing campaign that would eventually addict millions of Americans.
Purdue’s deceptive practices extended far beyond simple marketing claims. The company implemented a common “playbook” that included promoting false statements about the non-addictive nature of opioids, masking signs of addiction by labeling them as “pseudoaddiction” and encouraging greater opioid use to treat it, and suggesting that alternative pain relief methods were riskier than opioids. These grossly misleading claims were disseminated through a vast network of sales representatives who specifically targeted susceptible doctors and flooded medical publications with deceptive advertisements.
The scope of Purdue’s deception was so extensive that in 2007, the company and three executives pleaded guilty to criminal charges of misbranding OxyContin by falsely claiming it was less addictive and less subject to abuse than other opioids, resulting in $634 million in fines. However, by this time, the damage was already done—millions of Americans had been prescribed a highly addictive substance under false pretenses.
The Fifth Vital Sign: Institutionalizing Overprescription
The overprescription crisis was significantly amplified by the medical establishment’s adoption of “pain as the fifth vital sign” in the mid-1990s. Dr. James Campbell’s 1995 Presidential Address to the American Pain Society, which promoted treating pain as a vital sign, was rapidly adopted by the Veterans Health Administration and the Joint Commission on Accreditation of Healthcare Organizations.
While well-intentioned, this initiative created a perfect storm for opioid overprescription. Guided by pain as the fifth vital sign mandates, patients reported pain and expected providers to respond, but many clinicians lacked adequate education in pain evaluation and management. When non-opioid treatments failed to reduce reported pain scores, providers increasingly turned to opioid medications, contributing to a doubling of opioid dispensing rates.
The unintended consequences of treating pain as a vital sign became clear as prescription opioid use climbed alongside overdose deaths. By 2016, recognizing the role this initiative played in the opioid crisis, the American Medical Association voted to stop treating pain as the fifth vital sign. The organization concluded that this policy, combined with other factors including aggressive pharmaceutical marketing, had exacerbated the epidemic.
Geographic Targeting and Vulnerable Populations
Purdue Pharma’s marketing strategy deliberately targeted rural and economically disadvantaged communities where opioid addiction would take its heaviest toll. The marketing of prescription opioids such as OxyContin was particularly aggressive in rural communities, especially those surrounding Appalachia. These areas became epicenters of the opioid crisis, with higher prescription rates, greater availability for diversion, and more devastating community impacts.
Rural populations proved particularly vulnerable to opioid addiction due to multiple converging factors. Higher rates of physically demanding occupations led to more legitimate pain conditions requiring treatment. Limited healthcare options meant fewer alternatives to opioid therapy. Tight kinship and social networks facilitated faster diffusion of prescription opioids among those at risk, as family and friends became primary distribution sources.
The data reveal striking disparities in opioid prescribing between rural and urban areas. Patients in rural areas are 10 percentage points more likely to receive an opioid prescription, with roughly half of this differential attributable to underlying health differences and the remainder explained by supply and demand factors. This geographic targeting created concentrated addiction hotspots that devastated entire communities for generations.
The Prescription-to-Street Pipeline
The aggressive marketing and overprescription of opioids created a massive diversion pipeline that fed illicit drug markets. OxyContin’s controlled-release mechanism could be easily defeated by crushing the tablets, allowing users to extract the full dose of oxycodone for immediate absorption. Purdue’s own testing in 1995 had demonstrated that 68% of the oxycodone could be extracted from a crushed OxyContin tablet, yet the company proceeded with marketing despite knowing this vulnerability.
The regions of the country with the earliest and highest availability of prescribed OxyContin experienced the greatest initial abuse and diversion. As prescription opioids became widely available through legitimate medical channels, they created new pathways for addiction that hadn’t existed before. Family members, friends, and acquaintances became primary sources of diverted medications, fundamentally altering drug distribution networks.
The scale of this diversion was staggering. By 2004, OxyContin had become the most prevalent abused prescription opioid in the United States, with abuse rates directly correlating to prescription availability. The pharmaceutical industry had essentially created a new drug market by flooding communities with addictive substances promoted as safe.
Regulatory Failures and Systemic Breakdown
The opioid crisis represents what experts have termed “a multi-system failure of regulation,” with the FDA playing a central role in enabling the epidemic. The FDA failed to properly enforce the Food, Drug, and Cosmetic Act when approving Purdue Pharma’s new drug application for extended-release oxycodone. The agency accepted fraudulent claims about addiction risk without requiring adequate evidence, allowing dangerous marketing to proceed unchecked.
The regulatory failures extended beyond initial drug approval to ongoing oversight of marketing practices. Despite mounting evidence of abuse and addiction, regulatory agencies failed to take meaningful action to curb deceptive promotional activities. The FDA’s inadequate response allowed pharmaceutical companies to continue aggressive marketing campaigns that prioritized sales over safety for years after addiction problems became apparent.
Prescription Drug Monitoring Programs (PDMPs), designed to track controlled substance prescriptions and prevent doctor shopping, proved insufficient to address the scale of the crisis. While PDMPs were implemented in 49 states by 2018, their effectiveness remained limited by voluntary use, inadequate integration with healthcare systems, and insufficient real-time monitoring capabilities.
The Transition to Illicit Drugs
As policy makers and medical professionals began recognizing the dangers of opioid overprescription, efforts to reduce legitimate prescribing had unintended consequences that further complicated the crisis. Tightened prescribing guidelines, prescription duration limits, and increased monitoring led to scarcity of prescription opioids on the street, accelerating transitions to heroin and fentanyl.
Qualitative research with people who use opioids revealed how policy changes intended to reduce overprescribing inadvertently pushed users toward more dangerous substances. As doctors became less likely to prescribe or refill prescription opioids, users turned to heroin and fentanyl as more accessible alternatives. This transition likely increased risks of overdose and infectious disease transmission, creating new public health challenges.
The three waves of the opioid crisis illustrate this tragic progression. The first wave began with increased prescribing in the 1990s, leading to prescription opioid overdose deaths. The second wave emerged around 2010 with rapid increases in heroin deaths as prescription access tightened. The third wave began in 2013 with substantial increases in synthetic opioid deaths, particularly from illegally manufactured fentanyl.
Unprecedented Public Health Impact
The scale of the opioid crisis defies comprehension. Between 2011 and 2021, unintentional opioid toxicity deaths increased 289%, from 19,395 to 75,477 annually. By 2021, opioid toxicity was responsible for 10.2% of all deaths among those aged 15-19, 21.7% among those aged 20-29, and 21.0% among those aged 30-39. This means that more than one in five deaths among young adults now involves opioids (JAMA).
The total years of life lost due to opioid toxicity increased 276% over the study period, from 777,597 in 2011 to 2,922,497 in 2021. The COVID-19 pandemic dramatically accelerated these losses, with years of life lost increasing 62.9% between 2019 and 2021. Among individuals aged 15-39, the years of life lost from opioids exceeded those from COVID-19 in both 2020 and 2021, highlighting the distinct impact of the drug crisis on younger populations (JAMA).
The epidemic’s impact extends far beyond mortality statistics. In New York alone, opioid-related deaths grew by almost 300% between 2010 and 2020, comprising 85% of all drug overdose deaths by 2020. The crisis has disrupted families, overwhelmed healthcare systems, strained social services, and imposed enormous economic costs on communities nationwide.
Corporate Accountability and Legal Consequences
The legal reckoning for the opioid crisis has resulted in some of the largest corporate settlements in American history. The landmark $7.4 billion settlement with Purdue Pharma and the Sackler family represents the largest settlement to date with individuals responsible for the crisis. Under this agreement, the Sackler family will pay up to $6.5 billion over 15 years, while Purdue will contribute an additional $900 million.
Additional settlements have extracted billions more from pharmaceutical companies and distributors. The three largest drug distributors—McKesson, Cardinal Health, and AmerisourceBergen—agreed to pay up to $21 billion over 18 years, while Johnson & Johnson will pay up to $5 billion over nine years. These settlements total over $50 billion nationally, though this amount pales in comparison to the estimated $600 billion annual costs of the opioid crisis.
The settlements require more than financial payments. Companies must implement monitoring systems to detect suspicious orders, limit marketing practices, and submit to ongoing oversight. The Sackler family is permanently banned from manufacturing, selling, or marketing opioids in the United States. However, critics argue these penalties are insufficient given the scope of harm caused and the profits extracted.
Lessons Learned and Ongoing Challenges
The opioid crisis reveals fundamental flaws in how controlled substances are regulated, marketed, and prescribed. The pharmaceutical industry’s ability to manipulate medical practice through aggressive marketing, despite clear addiction risks, demonstrates the need for stronger regulatory oversight. The crisis shows how corporate profits can override public health when regulatory systems fail to adequately protect vulnerable populations.
Current efforts to address the crisis focus on multiple fronts. Enhanced prescription monitoring, improved integration of PDMPs with electronic health records, and evidence-based prescribing guidelines aim to prevent future overprescription. Expanded access to addiction treatment, including medication-assisted treatment with buprenorphine and methadone, addresses existing addiction. Harm reduction strategies such as naloxone distribution and safe consumption sites attempt to reduce overdose deaths.
However, significant challenges remain. The transition from prescription opioids to illicit fentanyl has created new dangers, as illegally manufactured fentanyl is far more potent and unpredictable than prescription medications. The integration of fentanyl into other drug supplies means even people using non-opioid substances face overdose risks. The COVID-19 pandemic has disrupted treatment access and increased social isolation, contributing to accelerating overdose rates.
Preventing Future Pharmaceutical-Induced Epidemics
The opioid crisis provides crucial lessons for preventing future pharmaceutical-induced public health disasters. Stronger FDA oversight of controlled substance marketing, including restrictions on promotional spending and sales representative activities, could prevent similar manipulation. Enhanced post-marketing surveillance and rapid response to emerging abuse patterns could catch problems earlier.
Medical education must better prepare healthcare providers to recognize and manage addiction risks. The pain as the fifth vital sign initiative demonstrates how well-intentioned policies can have catastrophic unintended consequences when implemented without adequate safeguards. Future pain management approaches must balance legitimate patient needs with addiction prevention.
Legal accountability mechanisms must be strengthened to ensure pharmaceutical companies face meaningful consequences for harmful marketing practices. The current settlement amounts, while substantial, may be viewed as acceptable business costs rather than meaningful deterrents. Criminal prosecutions of executives responsible for deceptive marketing could provide stronger incentives for corporate responsibility.
The Path Forward
Addressing the ongoing opioid crisis requires sustained commitment to evidence-based solutions across prevention, treatment, and harm reduction. This includes expanding access to addiction treatment, implementing comprehensive harm reduction programs, and addressing underlying social determinants that increase addiction vulnerability. Rural and economically disadvantaged communities that bore the heaviest burden of the crisis need targeted resources and support.
The crisis also demands continued vigilance against pharmaceutical industry practices that prioritize profits over public health. The lessons learned from OxyContin’s deceptive marketing must inform ongoing regulatory oversight of new controlled substances. Healthcare systems must develop better safeguards against corporate influence on medical practice, ensuring that treatment decisions are based on evidence rather than marketing.
Conclusion: A Preventable Tragedy
The opioid crisis stands as perhaps the clearest example in modern American history of how corporate malfeasance can create a public health catastrophe. The overprescription of OxyContin and other opioid painkillers was not an unfortunate side effect of medical progress—it was the predictable result of a calculated campaign to maximize profits while minimizing accountability.
The human cost of this deception continues to mount daily, with nearly 220 Americans dying from drug overdoses every day. Families across the country have been shattered, communities have been devastated, and an entire generation has been scarred by addiction that could have been prevented. The crisis has demonstrated that when corporate interests are allowed to override public health concerns, the consequences can be catastrophic and long-lasting.
While settlements and policy changes represent important steps toward accountability, they cannot restore the lives lost or undo the damage inflicted on millions of families. The true measure of society’s response to this crisis will be whether the lessons learned prevent similar pharmaceutical-induced epidemics in the future. This requires not only stronger regulations and oversight but also a fundamental shift in how we balance corporate freedom with public health protection.
The opioid crisis serves as a stark reminder that vigilance against corporate deception is essential to protecting public health. As new medications and marketing strategies emerge, regulators, healthcare providers, and communities must remain alert to the warning signs that preceded this devastating epidemic. Only by learning from this tragic chapter in American public health can we hope to prevent similar catastrophes and begin the long process of healing the communities that were harmed.