The world faces an unprecedented addiction crisis, with 292 million people using drugs and 64 million suffering from drug use disorders. Opioid addiction devastates the brain through neuroplastic changes that create lasting vulnerability, while genetic predisposition and early trauma increase susceptibility. As deaths mount—particularly in North America—urgent investment in novel treatments, including psychedelic therapies, offers hope for breaking the cycle.
Introduction
Addiction has become one of the most pressing global health crises of the 21st century. According to the World Drug Report 2024, drug use has increased by 20% over the past decade, with 292 million people using illicit substances worldwide. Among these, opioids represent the deadliest class of drugs, claiming hundreds of thousands of lives annually and fundamentally rewiring the human brain in ways that perpetuate dependency. This comprehensive examination explores the escalating addiction pandemic, focusing on the neurobiological devastation caused by opioids, the underlying vulnerability factors that predispose individuals to addiction, and the urgent need for innovative treatment approaches to address this crisis (unodc).
The Scale of the Global Crisis
The numbers paint a stark picture of humanity’s struggle with addiction. As of 2022, an estimated 64 million people worldwide suffer from drug use disorders, yet only one in eleven receives treatment. This treatment gap widens dramatically in developing regions, where coverage drops to just 2.8% in Africa and 5.1% in Asia. Cannabis remains the most widely used illicit drug with 228 million users, followed by opioids at 60 million users, amphetamines at 30 million, cocaine at 23 million, and ecstasy at 20 million.
The emergence of nitazenes—synthetic opioids potentially more potent than fentanyl—has compounded the crisis, resulting in increased overdose deaths across several high-income countries. These substances represent the latest evolution in an arms race between law enforcement and drug manufacturers, with each new synthetic compound becoming more dangerous and harder to detect.
The Opioid Epidemic: A Crisis Within a Crisis
Global Patterns and Mortality
Opioids continue to be the most lethal class of drugs globally, responsible for close to 80% of the estimated 600,000 drug-related deaths in 2019. The World Health Organization estimates that approximately 125,000 people died specifically from opioid overdose in 2019, though non-fatal overdoses occur several times more frequently. In Europe, 6,400 deaths involving illicit drugs were reported in 2022, with opioids involved in three-quarters of these fatalities.
The North American Catastrophe
North America bears the heaviest burden of the opioid crisis. In 2018 alone, approximately 68,500 Americans died from opioid overdoses involving prescription pain relievers, heroin, and synthetic opioids like fentanyl. The economic toll is staggering, with the crisis costing over $150 billion in the United States in 2015. Canada faces a parallel emergency, with 3,987 opioid-related deaths in 2017 and an estimated 17 Canadians hospitalized daily due to opioid poisoning.
The crisis has evolved through distinct phases. Initially driven by aggressive marketing of prescription opioids by pharmaceutical companies in the 1990s, it shifted toward heroin use as prescriptions became more restricted. Since 2013, synthetic opioids—particularly fentanyl and its analogs—have dominated the landscape. Fentanyl, which is 50-100 times more potent than morphine, now accounts for the majority of overdose deaths.
European Patterns and Emerging Threats
While Europe has experienced lower mortality rates than North America, concerning trends are emerging. In 2022, 163 fatal overdoses involved fentanyl in Europe, with Germany reporting 73 of these cases. The relatively low numbers may reflect underreporting rather than true prevalence, as detection systems struggle to keep pace with rapidly evolving synthetic drug markets.
The Neurobiology of Opioid Addiction
Mechanisms of Brain Hijacking
Opioids exert their effects by binding to mu-opioid receptors throughout the brain and body, particularly in regions involved in pain perception and reward processing. The ventral tegmental area (VTA) and nucleus accumbens—core components of the brain’s reward circuit—become dysregulated through chronic opioid exposure. This hijacking of natural reward pathways explains why individuals with opioid use disorder often lose interest in previously enjoyable activities, a phenomenon known as anhedonia.
Neuroplastic Changes and Brain Adaptation
The devastating impact of opioids extends far beyond acute intoxication. Chronic exposure triggers profound neuroplastic changes that restructure the brain’s architecture. Research has identified several categories of neuroplasticity associated with repeated opioid use:
- Immediate Response Adaptations: Acute opioid administration induces activity-dependent genes such as c-fos, Arc, and Homer1a. With repeated exposure, the brain develops tolerance to this genetic induction, requiring higher doses to achieve the same effect.
- Progressive Accumulation Changes: Proteins like deltaFosB gradually accumulate with repeated drug exposure and can persist for weeks or months. This accumulation is thought to mediate the transition from casual drug use to compulsive, relapsing patterns of use (nature).
- Structural Modifications: Chronic morphine exposure reduces dendritic spines on ventral tegmental area neurons and decreases neurogenesis in the hippocampus. These changes may underlie the cognitive impairments observed in patients receiving long-term opioid therapy (nih).
Myelination and Addiction Learning
Recent groundbreaking research from Stanford Medicine has revealed that adaptive myelination—the process by which active brain circuits gain more fatty insulation for faster signal transmission—plays a crucial role in addiction learning. A single dose of morphine can trigger myelination of dopamine-producing neurons, spurring animals to seek more of the drug. When this myelination process is blocked, drug-seeking behaviour disappears entirely.
This discovery illuminates how the same neuroplastic mechanisms essential for learning and memory can be co-opted by addictive substances. The brain literally optimizes itself for drug-seeking behavior, creating physical changes that persist long after acute drug effects have worn off.
Glutamate System Dysfunction
The glutamate system undergoes significant disruption in opioid addiction. Chronic opioid exposure leads to reduced glutamate levels in key brain regions and disrupts glutamate homeostasis. During withdrawal, there is a marked imbalance with reduced basal glutamate levels but potentiated release during drug-seeking episodes. This dysfunction contributes to the intense craving and compulsive drug-seeking that characterizes addiction (nih).
Vulnerability Factors: Who Is at Risk?
Genetic Predisposition
Genetics account for approximately 40-60% of addiction vulnerability, making it one of the most heritable psychiatric conditions. Heritability estimates for opioid addiction specifically range around 0.6-0.7, indicating a strong genetic component. Key genes implicated in addiction vulnerability include:
OPRM1: Encodes the mu-opioid receptor and influences individual responses to opioids. Variants in this gene affect both addiction risk and treatment response to medications like naltrexone.
DRD2: Codes for dopamine D2 receptors in the brain’s reward system. Variations influence receptor density and sensitivity to rewarding stimuli.
COMT: Affects dopamine metabolism in the prefrontal cortex, influencing executive function and impulse control.
The genetic architecture of addiction involves both substance-specific genes and genes affecting common pathways. For instance, variants in the CHRNA5-CHRNA3-CHRNB3 gene cluster influence dependence on multiple substances, though the direction of effects varies across different drugs (nih).
Early Life Trauma and Environmental Factors
Environmental factors, particularly early life adversity, interact powerfully with genetic predisposition to increase addiction risk. Childhood trauma, abuse, and neglect can epigenetically program stress-response genes, leading to heightened HPA-axis reactivity that persists into adulthood. This biological embedding of early adversity creates lasting vulnerability to stress-related disorders, including addiction.
The three-factor model of addiction vulnerability emphasizes the interaction between genetic factors, environmental influences, and repeated drug exposure. Even individuals with relatively low genetic risk can develop addiction under circumstances of high-dose, prolonged exposure to addictive substances.
The Cycle of Destruction: How Addiction Perpetuates Itself
Hypofrontality and Executive Dysfunction
Neuroimaging studies consistently demonstrate reduced prefrontal cortical activity in individuals with opioid use disorder. This “hypofrontality” impairs executive functions crucial for self-regulation, including working memory, cognitive flexibility, and inhibitory control. Paradoxically, when exposed to drug-related cues, these same regions show hyperactivation, correlating with craving intensity.
This creates a devastating paradox: individuals with addiction have both heightened sensitivity to drug cues and reduced capacity to resist them. The prefrontal cortex, which normally serves as the brain’s “brakes,” becomes compromised while the accelerator—the drug-sensitized reward system—becomes hypersensitive.
Stress Sensitivity and Relapse Vulnerability
Chronic opioid use dysregulates the body’s stress response systems, leading to heightened sensitivity to stressors that can trigger relapse months or years after achieving sobriety. The extended amygdala—a brain region involved in stress and negative emotional states—undergoes extensive neuroplastic changes that persist long after acute withdrawal. These changes contribute to the protracted withdrawal syndrome that makes long-term recovery so challenging.
Novel Treatment Approaches: The Promise of Psychedelic Medicine
Current Treatment Limitations
Traditional approaches to opioid addiction treatment include opioid agonist therapy (methadone, buprenorphine), antagonist medications (naltrexone), and psychosocial interventions. While these approaches can be effective, they often fall short of providing lasting recovery for many individuals. Relapse rates remain high, and many people struggle with treatment retention and adherence.
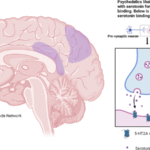
Psychedelics as Neuroplasticity Modulators
Emerging research suggests that psychedelic compounds may offer unique therapeutic benefits for addiction treatment by promoting beneficial neuroplastic changes. These substances, termed “psychoplastogens,” can rapidly promote dendritic growth and spine formation—potentially reversing some of the neural damage caused by chronic drug use.
Ibogaine: The Most Studied Psychedelic for Opioid Addiction
Ibogaine, derived from the African iboga plant, has shown remarkable promise in preclinical and anecdotal reports for treating opioid addiction. Animal studies demonstrate that ibogaine can reduce opioid self-administration, diminish withdrawal symptoms, and decrease conditioned place preference for opioids. The compound appears to work through multiple mechanisms, including effects on dopamine, serotonin, and glutamate systems.
However, ibogaine carries significant cardiovascular risks and requires careful medical supervision. Research into safer analogs like 18-methoxycoronaridine (18-MC) aims to preserve therapeutic benefits while reducing toxicity.
Psilocybin: Population-Level Evidence
Large-scale epidemiological studies provide compelling evidence for psilocybin’s potential in addiction treatment. Analysis of National Survey on Drug Use and Health data from 2015-2019 found that lifetime psilocybin use was associated with 30% lower odds of opioid use disorder. This association was robust across seven of eleven DSM-IV criteria for opioid dependence and abuse.
The University of Wisconsin-Madison has launched groundbreaking clinical trials investigating psilocybin for both opioid and methamphetamine use disorders—the first of their kind. These open-label studies will test safety and feasibility, potentially paving the way for larger controlled
Ketamine: Rapid-Acting Potential
Ketamine, an NMDA receptor antagonist, has shown promise in treating both depression and addiction. Its rapid antidepressant effects may be particularly valuable for individuals with co-occurring mood disorders and substance use problems. The drug appears to promote glutamate system neuroplasticity and may help restore the excitatory-inhibitory balance disrupted by chronic opioid use.
The Urgent Need for Research Investment
Current Research Gaps
Despite the enormous public health impact of addiction, research funding remains inadequate relative to the scope of the crisis. Key research priorities include:
Mechanistic Studies: Better understanding of how psychedelics promote therapeutic neuroplasticity and whether these changes can durably reverse addiction-related brain changes.
Clinical Trial Development: Large-scale, randomized controlled trials are needed to establish safety and efficacy of psychedelic treatments for addiction.
Personalized Medicine: Research into genetic and biomarker predictors of treatment response could help match individuals to optimal therapies.
Combination Approaches: Investigating how psychedelic treatments might be combined with traditional therapies and behavioral interventions.
Regulatory and Policy Considerations
The therapeutic potential of psychedelics requires careful regulatory frameworks that balance innovation with safety. Recent FDA designations of “breakthrough therapy” status for psilocybin in depression treatment provide a model for expedited development of addiction treatments.
Policymakers must also address the social determinants of addiction, including poverty, trauma exposure, and lack of healthcare access. Addiction is fundamentally a bio-psycho-social disorder that requires comprehensive, multi-level interventions.
Conclusion
The global addiction crisis, driven largely by opioid dependency, represents one of the most complex and devastating public health challenges of our time. The neurobiological understanding of addiction reveals how these substances hijack fundamental brain processes, creating lasting changes that perpetuate dependency long after acute effects subside. Genetic vulnerability and early life adversity interact to create differential risk, while the chronic nature of brain changes makes recovery particularly challenging.
However, emerging research into psychedelic therapies offers unprecedented hope for breaking the cycle of addiction. These compounds appear to work through novel mechanisms—promoting beneficial neuroplasticity and potentially reversing some addiction-related brain changes. As traditional approaches reach their limits, the careful development of psychedelic-assisted treatments may provide the breakthrough needed to address this crisis.
The path forward requires sustained research investment, thoughtful regulation, and a commitment to treating addiction as the complex brain disorder it truly is. Only through continued scientific innovation, combined with comprehensive public health approaches, can we hope to stem the tide of this devastating epidemic and restore hope to millions of affected individuals and families worldwide.
Trusted Resources for Further Reading:
- World Health Organization: Opioid Overdose Fact Sheet – https://www.who.int/news-room/fact-sheets/detail/opioid-overdose
- UNODC World Drug Report 2024 – https://www.unodc.org/unodc/press/releases/2024/June/unodc-world-drug-report-2024_-harms-of-world-drug-problem-continue-to-mount-amid-expansions-in-drug-use-and-markets.html
- European Drug Report 2024 – https://www.euda.europa.eu/publications/topic-overviews/drug-induced-deaths-faq_en
- Stanford Medicine Myelination Research – https://med.stanford.edu/news/all-news/2024/06/myelin-addiction.html
- University of Wisconsin Psilocybin Trials – https://news.wisc.edu/two-first-in-kind-clinical-trials-explore-psilocybin-for-substance-misuse/